


Get new exclusive access to healthcare business reports & breaking news




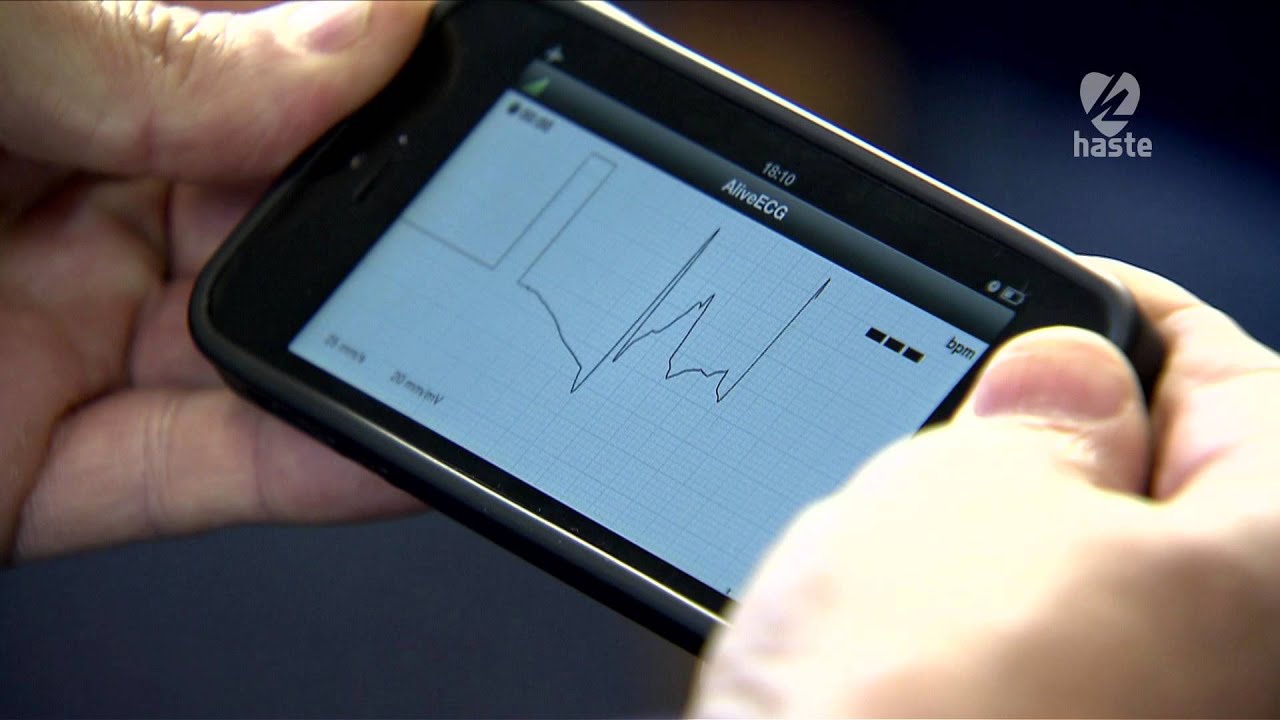
CardioComm Solutions, Inc., a Toronto, Canada-based company, is making data available on consumer smartphones with a new 510(k) clearance for its Mobile smartphone app called GEMS. The company also recently received clearance by the U.S. Food and Drug Administration (FDA) for their new ECG device, known as HeartCheck CardiBeat which works in tandem with the GEMS app. Each received clearance as a Class II medical device and both are available for direct to consumer sales. The smartphone app works on both iOS and Android devices. GEMS mobile is the only ECG management smartphone app that can be connected to several different monitoring devices.
CardioComm Solutions and its software allows data to be received and managed anywhere in the world using wireless devices, networks, and the internet to perform ECGs.
An ECG, also known as an electrocardiogram, records the electrical activity of the heart at rest. It provides information about heart rate and rhythm and shows any signs of hypertension (high blood pressure). ECGs are useful for individuals who have risk factors for heart disease, such as high blood pressure or symptoms of heart disease such as chest pain and palpitations. The CardioComm’s CardiBeat device is able to monitor a patient’s condition so any patient with a smartphone can be more consistently aware of risks and check blood pressure levels.
The Global ECG Management System (GEMS) mobile smartphone app supports Cardiocomm’s CardiBeat device. In a press release, the company stated that the app is a “slimmed” down version of GEMS that was designed for hospital use. In the press release, the company said “The Bluetooth enabled and rechargeable CardiBeat allows a medical grade ECG recording to be taken by holding the device in both hands or by holding the device in the right hand and against the left side of the chest”. Their second option is more accurate in diagnosing atrial fibrillation and atrial flutter, which are forms of arrhythmias.
HeartCheck CardiBeat is a handheld ECG device that is newly cleared by the FDA for consumer use. It is a pocket-sized PEN that allows for heart readings from anywhere and at anytime as soon as symptoms appear. It has many functions including providing monitoring systems for arrhythmias anywhere, allowing web access to qualified physicians without prescriptions being required. The HeartCheck PEN is compact, easy to use, and takes quick heart readings, in only 20 seconds. It also has the ability to store up to 20 heart rhythms and the data that is stored can be downloaded from a computer and sent to an expert or a physician for the ECG to be examined.
The CardioComm CardiBeat differs from previous devices in that it captures vital information in two ways. CardiBeat allows medical grade ECG recordings to be taken by simply holding the device in both hands or by holding it against the left side of the chest. Other ECG devices cannot obtain accurate information in quite these ways. Holding the device against the chest is more accurate than measurements made by previous devices when it comes to diagnosing arrhythmias (for example, atrial fibrillation and atrial flutter).
The app for the device allows for both post-event and continuous ECG monitoring. For an extra fee, patients have access to the paid service whereby individuals can have their ECGs examined and by experts.
This device is recommended for seniors and for use in nursing homes and home care facilities. It can also be used by athletes.
CardioComm Solutions Inc. participates in the provision of management software solutions and specializes in software engineering of computer-based ECG management reports and systems.
CardioComm sells many products, all of which are engineered to work with computer-based ECG management systems. Some of these products include: the GlobalCardio 12 Lead, GEMS, Win, and others. Their latest product is the recently FDA approved HeartCheck PEN handheld ECG Device. They announced a smartphone that was connected to a wearable ECG in 2016, but it never entered the market.
The company has been an expert in the smartphone connectivity industry over the years by using consumer smartphones to help transmit data required for monitoring remote patients.
By getting a nod from the FDA for the new smartphone app and ECG device, CardioComm is able to make their device readily available in the US market. It is especially useful for the elderly, athletes, and others who want to take control of their heart health. This device allows heart readings to be taken quickly and effectively. Moving into the smartphone connectivity space is a smart move by CardioComm since ECGs can be monitored remotely by physicians and clinics to make the experience easy for users.