


Get new exclusive access to healthcare business reports & breaking news




The Food and Drug Administration (FDA) just approved a new covered coronary stent device meant to treat perforations in the blood vessels of the heart.
Built by Biotronik, a Berlin-based company, the PK Papyrus stent platform, is the first FDA approved device of its kind in 17 years.
Here’s how it works.
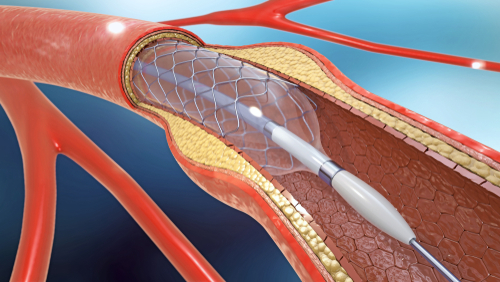
During a percutaneous coronary intervention (PCI), stents are inserted through an artery in the groin or wrist to enlarge a narrowing coronary artery and restore blood flow. PCI is a non-surgical procedure, which means patients recover faster and experience less pain than they would if they underwent heart surgery. However, rare complications such as deep tear in coronary arteries can still occur. The tear is referred to as an acute coronary artery perforation.
This is where Biotronik’s PK Papyrus ultrathin, covered stent comes into play.
Thanks to a unique polyurethane membrane that seals the tear from inside the artery, the device can stop blood from leaking out through the tear and collecting in the sac surrounding the heart. No open-heart surgery required.
“Perforation is very uncommon, but physicians need to be fully prepared for this emergency event,” said Marlou Janssen, president of Biotronik. “It’s unacceptable that this critical care area has seen no innovation in nearly two decades.”
There are fewer than 4,000 percutaneous coronary interventions per year in the United States that require a covered stent, making PK Papyrus a niche product. For this reason, the FDA classified the stent as a Humanitarian Use Device, a category reserved for medical devices intended to treat conditions that affect less than 8,000 Americans a year.
Approved in Europe since 2013, the new stent will be available to US physicians starting next year. So far, 80 patients received PK Papyrus stents and the results are promising: a 95 percent successful delivery and a 91 percent successful sealing of the perforation.
It’s worth noting that two deaths occurred during the original percutaneous coronary intervention and seven patients underwent treatment to drain fluids collected around the heart. Five patients died after the procedure has been successfully performed.
Biotronik’s device is contraindicated for those who can’t take anticoagulants or blood thinners and those with uncorrected bleeding disorders. While revolutionary in itself, this is not the company’s first FDA approved stent.
Its bare-metal PRO-Kinetic Energy system and Astron stents got the agency’s green light in 2017 and 2015 respectively.
The launch of PK Papyrus stent emphasises the growth of the implantable medical devices market. By 2024, this market will be worth almost $50 billion, a huge jump from from $32 billion in 2015.
Pacemakers, stents, and defibrillators are now in high demand in the U.S., as a result of increased obesity and high number of Baby Boomers reaching retirement age.
But it’s in the field of interventional cardiology where stents have made a significant impact. Coronary artery disease (CAD), the leading cause of death in the United States in both men and women, is now being treated without open heart surgery.
Instead, patients receive angioplasty surgery. First performed in the U.S. by cardiologist, Simon Stertzer in 1978, this non-invasive procedure relies on small balloon-tipped stent. Each year, about 600,000 Americans undergo angioplasty surgery, considerably more than those having a bypass.
What’s more, stents have proven to be great vehicles for stem cells, to treat heart failure after a heart attack.
Stents are also making their way into the pharma industry. Innovative drug-eluting stents (DES) help physicians control the release of drugs to coronary and peripheral arteries.
These devices will play a huge role in the advancement of precision medicine, which is believed to be the key to treating complex diseases. Several biopharmaceutical companies are expected to increase their investments in precision medicine by 33% over the next five years, shows a recent report.
Biotech companies are just starting to scratch the surface in terms of what stents can do. Despite safety concerns, bioresorbable stent technologies are in the process of being perfected. It might take another decade and countless clinical trials before these devices will reach their full medical potential, but once they do, a whole new world of possibilities will be opened for patients and doctors alike.